Scrambler Therapy® Technology ST-5A
Revolutionizing Pain Management
“Scrambler Therapy Technology System (ST-5A) – The Innovative International Patent Device”
-The science behind Scrambler Therapy Technology System (ST-5A), how it works, and the benefits it offers over other pain management devices.
-Exploring the indications and the medical conditions Scramble Therapy Technology can help treat.
-Information on where Scrambler Therapy Technology System (ST-5A) is available in renowned hospitals and medical centers across the United States, Europe, and around the world.
What is Scrambler Therapy Technology System ST-5A?
Scrambler Therapy Technology System ST-5A is an innovative pain management technology that uses electrical impulses to alleviate chronic pain. The system consists of a device that delivers low-level electrical impulses through electrodes placed on the skin, which effectively “scramble” the pain signals being sent to the brain. This process replaces painful signals with non-painful signals, and over time helps to reset the nerves and reduce or eliminate chronic pain.
The technology was developed by Professor Giuseppe Marineo in Italy and has been patented internationally. Scrambler Therapy Technology System ST-5A has been extensively studied and proven effective in managing various types of pain, including neuropathic pain, cancer pain, and chronic low back pain.

How it works and how it is different from other pain management devices?
“NeuroModulation“
Scrambler Therapy Technology System ST-5A works by sending electrical impulses to the nerves that are responsible for transmitting pain signals to the brain. The system uses a specific waveform that is designed to “scramble” the pain signals, essentially tricking the brain into perceiving nonpainful signals instead. This process is achieved by a process called “neuromodulation,” which helps to reset the nerves and reduce or eliminate chronic pain.
*The treatment usually requires cycles (that can be repeated), 10-12 treatments 0f 30 minutes, 5 days a week (two weeks in a row)

Device Description & The 5 Artificial Neurons
The Scrambler Therapy® Technology ST-5A is a patented non-invasive electro-analgesia device, with 5 channels that deliver low-intensity stimuli (max. 5 mA) generated by a completely automated treatment program. Five artificial neurons create “packets” (strings) of information recognized as “non pain”, the content of which is controlled by a proprietary algorithm. This various information of “no pain” produces an immediate analgesic effect, capable of completely eliminating pain in real time. Up to 5 pairs of independent electrodes can be placed on the patient’s body based on the need of the treatment. If the treatment is performed correctly, normally the pain is zeroed (or reduced very close to zero) in real time regardless of the initial intensity and the pathology that generated it. In this technology the concept of similarity that only considers the parameters of frequency, pulse width and intensity (used in other devices) is not applicable because they do not generate and do not characterize the specific information of “no pain” of the Scrambler Therapy®. In this sense any modification of the emissions of the Scrambler Therapy® in the form and organization of the flows in time, is functionally equivalent to the modification of the chemical formula of a drug.

What sets Scrambler Therapy Technology System ST-5A Apart From Others
What sets Scrambler Therapy Technology System ST-5A apart from other pain management devices is its unique waveform, which is unlike any other electrical stimulation therapy. Unlike other types of electrical stimulation, Scrambler Therapy Technology System ST-5A does not target muscle tissue, but rather the specific nerves responsible for transmitting pain signals. This makes it a much more targeted and effective therapy for chronic pain management.
Additionally, Scrambler Therapy Technology System ST-5A offers several advantages over other pain management devices. It is a non-invasive therapy, meaning that it does not require surgery or other invasive procedures. It is also non-addictive, has no risk of dependency or overdose, and has minimal side effects. This makes it a safe and effective alternative to traditional pain management treatments that rely on medication or invasive procedures.
The Scrambler Therapy device has since received FDA clearance in the United States for use in patients experiencing:
- Pain from cancer and chemotherapy
- Pain as a result of chronic diseases such as diabetes, multiple sclerosis
- Chronic back and neck pain
- Pain from failed back surgery syndrome
- Phantom limb pain.
Pain Management Benefits of Scrambler Therapy Technology System ST-5A
- Non-invasive: The therapy is non-invasive and does not require surgery or other invasive procedures, which reduces the risk of complications and downtime associated with invasive procedures.
- Safe: Scrambler Therapy Technology System ST5A is a safe therapy that has been extensively studied and shown to be effective in managing various types of pain. It has no risk of addiction or overdose and has minimal side effects.
- Effective: The therapy has been shown to be highly effective in managing chronic pain, including neuropathic pain, cancer pain, and chronic low back pain. It offers an alternative to traditional pain management treatments that may not be effective for some patients.
- Long-lasting relief: Scrambler Therapy Technology System ST-5A provides long-lasting relief for chronic pain. Unlike medications that require repeated dosing, Scrambler Therapy Technology System ST-5A offers long-term pain relief that can last for months or even years.
- Improved quality of life: Chronic pain can have a significant impact on a patient’s quality of life, causing physical, emotional, and social difficulties. Scrambler Therapy Technology System ST-5A offers relief from pain and can improve a patient’s overall quality of life.
- Cost-effective: Scrambler Therapy Technology System ST5A can be a cost-effective alternative to traditional pain management treatments that can be expensive and require ongoing medical care.

Clinically Proven and Recommended Indications for Scrambler Therapy Technology System
Indications for Scrambler Therapy Technology System ST-5A
“as per FDA”
- Non-invasive: The therapy is non-invasive and does not require surge
Indications of use
* Symptomatic relief of chronic, intractable pain, post-surgical and post-traumatic acute pain.
* Symptomatic relief of acute pain.
* Symptomatic relief of post-operative pain.Indications of use that derive from the scientific literature indexed and published in peer review scientific journals, based exclusively on this technology, are:
* Symptomatic relief of chronic opioid-resistant pain.
* Symptomatic relief of chronic neuropathic and oncological pain. In general, this device in the clinical studies is indicated for forms of severe chronic pain, in particular that which does not respond to other pharmacological and non-pharmacological treatments, including opioids.Scrambler Therapy Technology System ST-5A is used to manage various types of chronic pain, including neuropathic pain, cancer pain, and chronic low back pain. Here is some information on the indications for Scrambler Therapy Technology System ST-5A:
* Neuropathic pain: Scrambler Therapy Technology System ST-5A is particularly effective in managing neuropathic pain, which is caused by damage or dysfunction of the nervous system. This type of pain can be caused by a variety of conditions, including diabetes, multiple sclerosis, and nerve damage resulting from surgery or injury.
* Cancer pain: Scrambler Therapy Technology System ST-5A is also effective in managing cancer pain. Cancer pain can be caused by the tumor itself or by cancer treatments, such as chemotherapy or radiation therapy. Scrambler Therapy Technology System ST-5A can help reduce cancer pain and improve a patient’s quality of life.
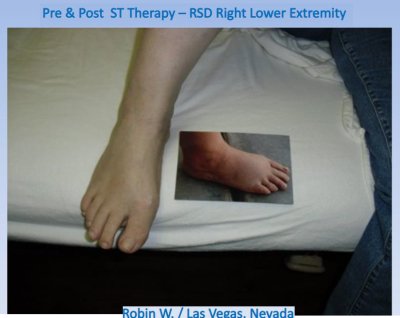
* Chronic low back pain: Scrambler Therapy Technology System ST-5A can also be used to manage chronic low back pain. Chronic low back pain is a common condition that affects millions of people worldwide. Scrambler Therapy Technology System ST-5A can help reduce pain and improve mobility in patients with chronic low back pain.Reflex Sympathetic Dystrophy (RSD) Syndrome: RSD is an older term used to describe one form of Complex Regional Pain Syndrome (CRPS). Both RSD and CRPS are chronic conditions characterized by severe burning pain, most often affecting one of the extremities (arms, legs, hands, or feet)
Reflex Sympathetic Dystrophy (RSD) Syndrome: RSD is an older term used to describe one form of
Complex Regional Pain Syndrome (CRPS). Both RSD and CRPS are chronic conditions characterized
by severe burning pain, most often affecting one of the extremities (arms, legs, hands, or feet)

Comparison and Advantages to Other Available Pain Management Treatments and Devices
Scrambler Therapy Technology System ST-5A has several advantages compared to other available pain management treatments and devices. Here are some points of comparison:
- Non-invasive: Scrambler Therapy Technology System ST-5A is a non-invasive pain management treatment that does not require any surgery or medication. It is a safe and effective alternative to invasive procedures that can be costly and have potential side effects.
- No known side effects: Scrambler Therapy Technology System ST-5A has no known side effects. This is in contrast to other pain management treatments and devices, such as opioids, which can cause a variety of side effects, including nausea, constipation, and addiction.
- Long-lasting pain relief: Scrambler Therapy Technology System ST-5A can provide long-lasting pain relief. This is because the therapy works by changing the way the brain processes pain signals, which can result in lasting pain relief even after the therapy is complete. Other pain management treatments, such as medications, often provide temporary pain relief that requires ongoing use.
- Versatile: Scrambler Therapy Technology System ST-5A can be used to manage a wide range of chronic pain conditions, including neuropathic pain, cancer pain, and chronic low back pain. This makes it a versatile treatment option that can be used to address different types of pain.
Renowned Hospitals and Medical Centers Where Scrambler Therapy Technology System ST 5A is Available in The USA
Scrambler Therapy Technology System ST-5A is becoming increasingly available in hospitals and medical centers around the world. Here are some examples of renowned hospitals and medical centers where it is currently available:
– MD Anderson Cancer Center in Houston, Texas, USA. Contact information: Phone: 1-877-632-6789, website: www.mdanderson.org.
– Mayo Clinic in Rochester, Minnesota, USA. Contact information: Phone: 1-507-284-2511, website: www.mayoclinic.org.
– Memorial Sloan Kettering Cancer Center in New York, New York, USA. Contact information: Phone: 1-800-525-2225, website: www.mskcc.org.
– University of California San Francisco (UCSF) Medical Center in San Francisco, California, USA. Contact information: Phone: 1-415-476-1000, website: www.ucsfhealth.org.
– Johns Hopkins Medicine in Baltimore, Maryland, USA. Contact information: Phone: 1-410-955-5000, website: www.hopkinsmedicine.org.
– University of Texas Southwestern Medical Center in Dallas, Texas, USA. Contact information: Phone: 1-214-645-5555, website: www.utsouthwestern.edu.
– Cleveland Clinic in Cleveland, Ohio, USA. Contact information: Phone: 1-216-444-2200, website: www.clevelandclinic.org.
– Karmanos Cancer Institute in Detroit, Michigan, USA. Contact information: Phone: 1-800-KARMANOS, website: www.karmanos.org.
– University of Michigan Health System in Ann Arbor, Michigan, USA. Contact information: Phone: 1-734-936-4000, website: www.uofmhealth.org.
– Indiana University Health in Indianapolis, Indiana, USA. Contact information: Phone: 1-317-944-5000, website: www.iuhealth.org
Renowned Hospitals and Medical Centers Where Scrambler Therapy Technology System ST-5A is Available in Italy
IRCCS Ospedale Pediatrico Bambino Gesù (Rome, Italy)
Unità Operativa di Anestesia e Rianimazione
Dir.Dott. Nicola Pirozzi
Piazza Sant’Onofrio 4-00165 – Roma
Responsabile Ambulatorio Scrambler Therapy
Dott.ssa Caterina Tomasello
Clinica European Hospital – Roma
Centro di Terapia del Dolore
Responsabile: dott. Riccardo Barchetta
Via Portuense, 700 – Roma
Azienda Ospedaliera Ospedale Niguarda Ca’ Granda – Milano
Piazza Ospedale Maggiore 3
20162 Milano
Terapia del dolore
Responsabile Dott. Paolo Notaro
Policlinico di Milano, Centro di medicina del dolore Mario Tiengo
Via della Commenda 19
20148 Milano
Medico responsabile della Scrambler Therapy
Dott. Vittorio Iorno
Azienda Ospedale Universitaria -Parma Via Gramsci 14
43126 Parma
Presso la Struttura di Alta Specializzazione di Terapia del Dolore
2° Anestesia, rianimazione e terapia antalgica Professor GUIDO FANELLI
Responsabile Ambulatorio Scrambler Therapy Dottor Christian Compagnone
Azienda Ospedaliera Careggi – Firenze
Sod Cure Palliative terapia del dolore
Presso Padiglione Cliniche chirurgiche (2° Piano) Via delle Oblate 1
50134 Firenze
Dottor Rocco Domenico Mediati
Responsabile Medico
Dottor Renato Vellucci
Azienda Ospedaliera di Rilievo Nazionale A. Cardarelli – Napoli
UOC Fisiopatologia e Terapia del Dolore e Cure Palliative
Via A. Cardarelli 9
80131 Napoli
Dott. Vincenzo Montrone
Scrambles and other types of treatment.
References
- Marineo G. Scrambler therapy for the treatment of chronic pain. Br J Anaesth. 2015 Jan;114(1):1-3.
- Marineo G. Untreatable pain resulting from abdominal cancer: new hope from biophysics? JOP. 2003 Sep;4(5 Suppl):182-6.
- Ricci M, Pirotti S, Scarpi E, et al. Scrambler therapy: a retrospective multicenter study on 136 patients with chronic neuropathic pain. Pain Pract. 2015 May;15(4):293-301.
- Sabato AF, Marineo G, Gatti A. Scrambler therapy. Minerva Anestesiol. 2005 Oct;71(10):479-82.
- Caro XJ, Winter EF. Scrambler therapy for fibromyalgia: a randomized, controlled trial. Pain Practice. 2016 Apr;16(4):431-41.
- Vigneri S, Gatti A, Pace MC, et al. Scrambler therapy for the treatment of chronic low back pain: a randomized controlled trial. Pain Physician. 2018 Jan;21(1):41-52.
- Ricci M, Bernabei M, Serrao G, et al. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: a preliminary study. J Palliat Med. 2018 Aug;21(8):1131-6.
- Liew WK, Kangas M, Zhang J, et al. Scrambler therapy for neuropathic pain secondary to spinal cord injury: a case series. J Spinal Cord Med. 2018 Mar;41(2):232-6.
- Giuseppe Marineo. Scrambler therapy: what is changing. Scrambler Therapy. https://www.scramblertherapy.org/scrambler-therapy-what-is-changing. Accessed March 5, 2023.
- New and important articles are to be published on Scramble Therapy Written by Johns Hopkins University and the Mayo Clinic by spring 2023 in The New England Journal of Medicine, The Lancet and JAMA.
About Scrambler Therapy®
Scrambler Therapy® scientific research and development technology has been developed in Italy by Professor Giuseppe Marineo, who is the sole owner of its intellectual property rights. The official “Scrambler Therapy”® scientific and clinical information website is:
www.scramblertherapy.org/
SOURCE Delta International Services & Logistics s.r.l.
Related Links
Clinical Practice Area (scramblertherapy.org)
Miti Infondati sulla Scrambler Therapy®
www.scramblertherapy.org/miti-sulla-scrambler-therapy
https://www.st-team.eu/
Clinical Studies Published (Update January 10, 2023)
research was carried out in the databases from PubMed/Medline.
Note: the proper use of Scrambler Therapy, being operator-dependent, allows only for a partial double-blind or single-blind trial design. Attempts to do a complete double-blind clinical trial automatically cause substantial changes in the standard treatment protocol, which requires substantial patient interaction to determine proper placements of electrodes and intensity of treatment. These changes prevent the operator to follow the normal procedures registered in the healthcare authorizations and can erase or significantly reduce the efficacy of the treatment, consequently invalidating the scientific data.
Scientific articles of reference (theory, methodology, clinic)
Marineo G. Inside the Scrambler Therapy, a Noninvasive Treatment of Chronic Neuropathic and Cancer Pain: From the Gate Control Theory to the Active Principle of Information. Integr Cancer Ther. 2019 Jan-Dec;18:1534735419845143. doi: 10.1177/1534735419845143. PMID: 31014125; PMCID: PMC6482660. Marineo G.
Untreatable pain resulting from abdominal cancer: new hope from biophysics? JOP. 2003 Jan;4(1):1-10. PMID: 12555009.
Review
- Karri J, Marathe A, Smith TJ, Wang EJ. The Use of Scrambler Therapy in Treating Chronic Pain Syndromes: A Systematic Review. Neuromodulation. 2022 Jun 9:S1094-7159(22)00681-X. doi: 10.1016/j.neurom.2022.04.045. Epub ahead of print. PMID: 35691908.
- Wang EJ, Limerick G, D’Souza RS, Lobner K, Williams KA, Cohen SP, Smith TJ. Safety of Scrambler Therapy: A Systematic Review of Complications and Adverse Effects. Pain Med. 2022 Sep 7:pnac137. doi: 10.1093/pm/pnac137. Epub ahead of print. PMID: 36069623.
- Nair A. Scrambler therapy: An opioid-sparing, non-invasive modality for chronic pain in patients. Saudi J Anaesth. 2022 Oct-Dec;16(4):525-526. doi: 10.4103/sja.sja_366_22. Epub 2022 Sep 3. PMID: 36337412; PMCID: PMC9630698.
- Wang EJ, Berninger LE, Komargodski O, Smith TJ. Painful Diabetic Neuropathy – Spinal Cord Stimulation, Peripheral Nerve Stimulation, Transcutaneous Electrical Nerve Stimulation, and Scrambler Therapy: A Narrative Review. Pain Physician. 2022 Nov;25(8):E1163-E1173. PMID: 36375183.
- Abdi S, Chung M, Marineo G. Scrambler therapy for noncancer neuropathic pain: a focused review. Curr Opin Anaesthesiol. 2021 Dec 1;34(6):768-773. doi: 10.1097/ACO.0000000000001073. PMID: 34653073
- Majithia N, Smith TJ, Coyne PJ, Abdi S, Pachman DR, Lachance D, Shelerud R, Cheville A, Basford JR, Farley D, O’Neill C, Ruddy KJ, Sparadeo F, Beutler A, Loprinzi CL. Scrambler Therapy for the management of chronic pain. Support Care Cancer. 2016 Jun;24(6):2807-14. doi: 10.1007/s00520-016-3177-3. Epub 2016 Apr 4. PMID: 27041741; PMCID: PMC4973603.
Randomized Controlled Trial
- Kashyap K, Singh V, Dwivedi SN, Gielen J, Bhatnagar S. Scrambler Therapy Enhances Quality of Life in Cancer Patients in a Palliative Care Setting: A Randomised Controlled Trial. Indian J Palliat Care. 2022 Jul-Sep;28(3):287-294. doi: 10.25259/IJPC_94_2021. Epub 2022 Jul 13. PMID: 36072252; PMCID: PMC9443118.
- Lee SY, Park CH, Cho YS, Kim L, Yoo JW, Joo SY, Seo CH. Scrambler Therapy for Chronic Pain after Burns and Its Effect on the Cerebral Pain Network: A Prospective, Double-Blinded, Randomized Controlled Trial. J Clin Med. 2022 Jul 22;11(15):4255. doi: 10.3390/jcm11154255. PMID: 35893347; PMCID: PMC9332864.
- Note: the proper use of Scrambler Therapy, being operator-dependent, allows only for a partial double-blind or single-blind trial design.
- Childs DS, Le-Rademacher JG, McMurray R, Bendel M, O’Neill C, Smith TJ, Loprinzi CL. Randomized Trial of Scrambler Therapy for ChemotherapyInduced Peripheral Neuropathy: Crossover Analysis. J Pain Symptom Manage. 2021 Jun;61(6):1247-1253. doi: 10.1016/j.jpainsymman.2020.11.025. Epub 2020 Nov 27. PMID: 33249081.
- Kashyap K, Singh V, Mishra S, Dwivedi SN, Bhatnagar S. The Efficacy of Scrambler Therapy for the Management of Head, Neck and Thoracic Cancer Pain: A Randomized Controlled Trial. Pain Physician. 2020 Sep;23(5):495-506. PMID: 32967392.
- Mealy MA, Kozachik SL, Cook LJ, Totonis L, Salazar RA, Allen JK, Nolan MT, Smith TJ, Levy M. Scrambler therapy improves pain in neuromyelitis optica: A randomized controlled trial. Neurology. 2020 May 5;94(18):e1900-e1907. doi: 10.1212/WNL.0000000000009370. Epub 2020 Apr 8. PMID: 32269109; PMCID: PMC7274926.
- Loprinzi C, Le-Rademacher JG, Majithia N, McMurray RP, O’Neill CR, Bendel MA, Beutler A, Lachance DH, Cheville A, Strick DM, Black DF, Tilburt JC, Smith TJ. Scrambler therapy for chemotherapy neuropathy: a randomized phase II pilot trial. Support Care Cancer. 2020 Mar;28(3):1183-1197. doi: 10.1007/s00520-019-04881-3. Epub 2019 Jun 17. PMID: 31209630.
- Marineo G, Iorno V, Gandini C, Moschini V, Smith TJ. Scrambler therapy may relieve chronic neuropathic pain more effectively than guidelinebased drug management: results of a pilot, randomized, controlled trial. J Pain Symptom Manage. 2012 Jan;43(1):87-95. doi: 10.1016/j.jpainsymman.2011.03.015. Epub 2011 Jul 16. PMID: 21763099.
Clinical Trial
- Tomasello C, Pinto RM, Mennini C, Conicella E, Stoppa F, Raucci U. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: A preliminary study. Pediatr Blood Cancer. 2018 Jul;65(7):e27064. doi: 10.1002/pbc.27064. Epub 2018 Apr 6. PMID: 29630779.
- Coyne PJ, Wan W, Dodson P, Swainey C, Smith TJ. A trial of Scrambler therapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother. 2013 Dec;27(4):359-64. doi: 10.3109/15360288.2013.847519. Epub 2013 Oct 21. PMID: 24143893; PMCID: PMC4378693.
- Kashyap K, Joshi S, Vig S, Singh V, Bhatnagar S. Impact of Scrambler Therapy on Pain Management and Quality of Life in Cancer Patients: A Study of Twenty Cases. Indian J Palliat Care. 2017 Jan-Mar;23(1):18-23. doi: 10.4103/0973-1075.197948. Erratum in: Indian J Palliat Care. 2017 Apr-Jun;23 (2):217. PMID: 28216858; PMCID: PMC5294432.
- Lee SC, Park KS, Moon JY, Kim EJ, Kim YC, Seo H, Sung JK, Lee DJ. An exploratory study on the effectiveness of “Calmare therapy” in patients with cancer-related neuropathic pain: A pilot study. Eur J Oncol Nurs. 2016 Apr;21:1-7. doi: 10.1016/j.ejon.2015.12.001. Epub 2015 Dec 29. PMID: 26952672.
Observational – Prospective Study
- Ricci M, Fabbri L, Pirotti S, Ruffilli N, Foca F, Maltoni M. Scrambler therapy: what’s new after 15 years? The results from 219 patients treated for chronic pain. Medicine (Baltimore). 2019 Jan;98(2):e13895. doi: 10.1097/MD.0000000000013895. PMID: 30633163; PMCID: PMC6336541.
- Pachman DR, Weisbrod BL, Seisler DK, Barton DL, Fee-Schroeder KC, Smith TJ, Lachance DH, Liu H, Shelerud RA, Cheville AL, Loprinzi CL. Pilot evaluation of Scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2015 Apr;23(4):943- 51. doi: 10.1007/s00520-014-2424-8. Epub 2014 Sep 24. PMID: 25245776; PMCID: PMC4383262.
- Compagnone C, Tagliaferri F; Scrambler Therapy Group. Chronic pain treatment and scrambler therapy: a multicenter retrospective analysis. Acta Biomed. 2015 Sep 14;86(2):149-56. PMID: 26422429.Acta Biomed
- Notaro P, Dell’Agnola CA, Dell’Agnola AJ, Amatu A, Bencardino KB, Siena S. Pilot evaluation of scrambler therapy for pain induced by bone and visceral metastases and refractory to standard therapies. Support Care Cancer. 2016 Apr;24(4):1649-54. doi: 10.1007/s00520-015-2952-x. Epub 2015 Sep 26. PMID: 26408323.
- Smith TJ, Marineo G. Treatment of Postherpetic Pain With Scrambler Therapy, a Patient-Specific Neurocutaneous Electrical Stimulation Device. Am J Hosp Palliat Care. 2018 May;35(5):812-813. doi: 10.1177/1049909113494002. Epub 2013 Jul 8. PMID: 23838448.
- Ko YK, Lee HY, Lee WY. Clinical experiences on the effect of scrambler therapy for patients with postherpetic neuralgia. Korean J Pain. 2013 Jan;26(1):98-101. doi: 10.3344/kjp.2013.26.1.98. Epub 2013 Jan 4. PMID: 23342218; PMCID: PMC3546221.
- Ghatak RK, Nandi SN, Bhakta A, Mandal GC, Bandyopadhyay M, Kumar S. Prospective study of application of biological communication (cybernatics) in management of chronic low back pain–a preliminary report. Nepal Med Coll J. 2011 Dec;13(4):257-60. PMID: 23016475.
- Sabato AF, Marineo G, Gatti A. Scrambler therapy. Minerva Anestesiol. 2005 Jul-Aug;71(7-8):479-82. PMID: 16012423.
Case Reports
- Murphy T, Erdek M, Smith TJ. Scrambler Therapy for the Treatment of Pain in Schwannomatosis. Cureus. 2022 Mar 13;14(3):e23124. doi: 10.7759/cureus.23124. PMID: 35464572; PMCID: PMC9001870.
- Wang EJ, Berninger LE, Pantelyat AY, Hunsberger JB, Smith TJ. Scrambler Therapy for the Treatment of Multiple System Atrophy-Parkinsonian Subtype Pain: A Case Report. A A Pract. 2022 Jan 18;16(1):e01560. doi: 10.1213/XAA.0000000000001560. PMID: 35050906.
- Murphy TK, Pardo CA, Roda RH, Stone RL, Smith TJ. Successful Treatment of Paraneoplastic Neuropathy and Pruritis With Scrambler Therapy: A Case Report. Cureus. 2022 Jul 14;14(7):e26861. doi: 10.7759/cureus.26861. PMID: 35978756; PMCID: PMC9375651.
- Christo PJ, Kamson DO, Smith TJ. Treatment of Déjerine-Roussy syndrome pain with scrambler therapy. Pain Manag. 2020 May;10(3):141-145. doi: 10.2217/pmt-2019-0065. Epub 2020 May 12. PMID: 32394815.Pain Manag
- Ahuja D, Bharati SJ, Gupta N, Kumar V, Bhatnagar S. Scrambler therapy: A ray of hope for refractory chemotherapy-induced peripheral neuropathy. Indian J Cancer. 2020 Jan-Mar;57(1):93-97. doi: 10.4103/ijc.IJC_361_18. PMID: 32129300.
- Yarchoan M, Naidoo J, Smith TJ. Successful Treatment of Scar Pain with Scrambler Therapy. Cureus. 2019 Oct 14;11(10):e5903. doi: 10.7759/cureus.5903. PMID: 31777690; PMCID: PMC6853271.
- Mealy MA, Newsome SD, Kozachik SL, Levy M, Smith TJ. Case Report: Scrambler Therapy for Treatment-Resistant Central Neuropathic Pain in a Patient with Transverse Myelitis. Int J MS Care. 2019 Mar-Apr;21(2):76-80. doi: 10.7224/1537-2073.2017-083. PMID: 31049038; PMCID: PMC6489430.
- Fabbri L, Pirotti SP, Rosati M, Ruffilli N, Maltoni M, Ricci M. Phantom limb pain successfully treated with scrambler therapy. Minerva Anestesiol. 2018 May;84(5):642-643. doi: 10.23736/S0375-9393.18.12490-4. Epub 2018 Jan 17. PMID: 29343050.
- D’Amato SJ, Mealy MA, Erdek MA, Kozachik S, Smith TJ. Scrambler Therapy for the Treatment of Chronic Central Pain: A Case Report. A A Pract. 2018 Jun 15;10(12):313-315. doi: 10.1213/XAA.0000000000000695. PMID: 29293482.
- Smith T, Cheville AL, Loprinzi CL, Longo-Schoberlein D. Scrambler Therapy for the Treatment of Chronic Post-Mastectomy Pain (cPMP). Cureus. 2017 Jun 21;9(6):e1378. doi: 10.7759/cureus.1378. PMID: 28775918; PMCID: PMC5522013.
- Raucci U, Tomasello C, Marri M, Salzano M, Gasparini A, Conicella E. Scrambler Therapy(®) MC-5A for Complex Regional Pain Syndrome: Case Reports. Pain Pract. 2016 Sep;16(7):E103-9. doi: 10.1111/papr.12474. Epub 2016 Jul 2. PMID: 27370908.
- Smith TJ, Auwaerter P, Knowlton A, Saylor D, McArthur J. Treatment of human immunodeficiency virus-related peripheral neuropathy with Scrambler Therapy: a case report. Int J STD AIDS. 2017 Feb;28(2):202-204. doi: 10.1177/0956462416656688. Epub 2016 Jul 10. PMID: 27330020.
